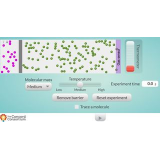
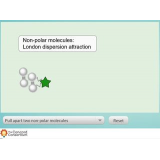
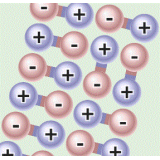
Online labs provide students with the possibility to conduct scientific experiments in an online environment. Remotely-operated labs (remote labs) offer an opportunity to experiment with real equipment from remote locations. Virtual labs simulate the scientific equipment. Data sets present data from already performed lab experiments. Please use the filters on the right to find appropriate online labs for your class.
Please note that the Go-Lab Authoring Platform Graasp is no longer maintained. This means that it is not possible to create and publish new Go-Lab Inquiry Learning Spaces using the labs listed on this page. However, you can still access the labs and use them directly on the providers' websites with help of the preview links, which you will find on the dedicated lab pages. If you are interested in creating and using Inquiry Learning Spaces in your classroom, please visit the new Authoring Platform Graasp.org
If you are looking for online labs selected for the curricula of Benin, Kenya or Nigeria, please visit our Collections page.